Controversial US study on hepatitis B vaccines in Africa is cancelled
AI Summary
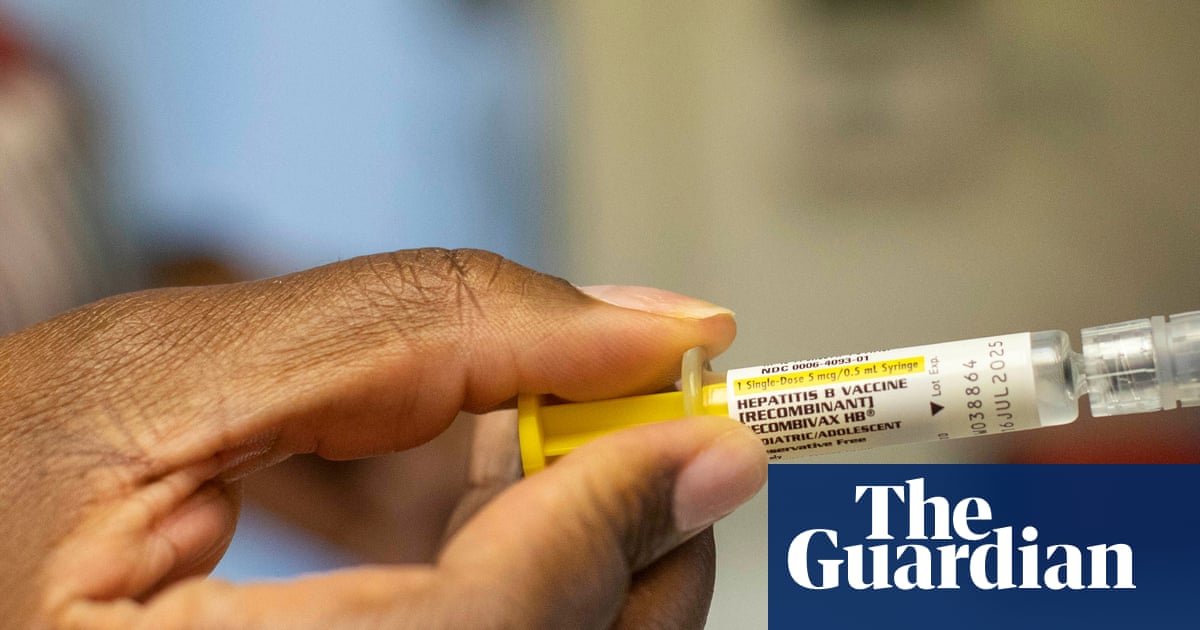
A controversial US-funded study on hepatitis B vaccines in Guinea-Bissau has been cancelled, according to the Africa Centres for Disease Control and Prevention (CDC). The $1.6 million study, funded under the purview of Robert F Kennedy Jr., faced criticism for ethical concerns regarding the withholding of proven hepatitis B vaccines from newborns in a country with a high disease burden. The Africa CDC halted the study due to its problematic design and ethical issues. While officials in Guinea-Bissau expressed interest in continuing the trial, the Africa CDC stated it would only proceed if redesigned to meet ethical standards. The US Department of Health and Human Services (HHS) did not respond to requests for comment.
Key Entities & Roles
Keywords
Sentiment Analysis
Source Transparency
This article was automatically classified using rule-based analysis. The political bias score ranges from -1 (far left) to +1 (far right).
Topic Connections
Explore how the topics in this article connect to other news stories